At the end of this lecture you should:
Surprisingly, it is only in the last 50 years that we have begun to understand the nature of the biological events which determine our sex, (and for that matter, why we bother with sex at all and why two sexes are better than three or more). It is not so long ago that women were blamed if they failed to produce a son for their husband and clearly it was thought that the power of sex determination lay within the body of the woman. During this century the chromosomal basis of human sex determination has been demonstrated and in the last few years some of the genes responsible have been identified.
The sexual identity of an individual is determined at several levels, chromosomal sex, gonadal sex, somatic sex and sexual orientation.
The chromosomal basis of sex determination in humans was recognized when metaphase chromosomes from dividing male and female cells could be studied and counted. The normal karyotype contains 46 chromosomes including either two X chromosomes (46XX, females) or one X chromosome and one Y chromosome (46XY, males). This seems superficially similar to the situation in Drosophila melanogaster where females have two sets of autosomes and two X chromosomes (2AXX) whereas males are 2AXY. However we see a profound difference when we examine individuals with abnormal chromosome complements.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Humans with 45X or 47XXX karyotypes are female and those with 47XXY karyotype are male. Therefore it can be deduced that the Y chromosome is sex determining in contrast to the Drosophila mechanism where every cell in the fly's body continually monitors the ratio of the number of X chromosomes to the number of haploid sets of autosomes, a ratio of 1:1 gives a female cell and 1:2 gives a male cell. Flies which have lost one X chromosome from some of their cells will be a patchwork of part male and part female tissue ( a gynandromorph).
Experiments carried out by Jost in the 1960s involving the removal of the embryonic gonad have revealed that in mammals, no matter what the chromosomal sex of the somatic cells, the body will develop as a female unless a male gonad is present to secrete the hormones Mullerian inhibiting substance, MIS, (also known as Anti Mullerian Hormone, AMH) and testosterone. This can be partially mimicked in the genetic condition testicular feminisation in which the gene coding for the androgen receptor is not expressed so that, although the testis in an XY individual secretes testosterone, the somatic tissues are unable to respond to it. Consequently the individual's body develops as a woman but with internal testes instead of ovaries. Several well known, voluptuous film stars have been rumoured to have this condition. A good web site to visit is Complete Androgen Insensitivity Syndrome at the University of Melbourne. Another is that of The Androgen Insensitivity Syndrome Support Group.
Click here for a diagram, taken from the University of Melbourne site, which shows in outline the regression of the Mullerian ducts and the development of the Wolffian tubules in response to MIS and androgen.
The embryonic gonad is comprised of four types of cell. In the mouse the structure is known as the germinal ridge. Initially the germ cells themselves are not present in the germinal ridge, they migrate to it from the yolk sac where they sought sanctuary during the upheaval of gastrulation. In mice homozygous for the mutation Steel this migration fails to occur, nevertheless the differentiation into testis in an XY individual still occurs. So germ cells are not necessary for correct differentiation. Transplantation experiments, in which male germ cells are transplanted to a female gonad, bear this out. The male germ cells in a female environment begin to develop as oocytes.
The gonad is indifferent up to 11.5 dpc, by 12.5 dpc Sertoli cells are visible in males and Leydig cells shortly after. There is no visible change in these cells in the ovary until much later. Some of the connective tissue cells have organised into a testis specific pattern by 12.5 dpc. (See Martineau et al)
| germ cells | supporting cells | steroidogeneic cells | connective cells | |
| male | respond to environment | Sertoli cells | Leydig cells | essentially the same in each sex |
| female | respond to environment | Follicle (granulosa) cells | Theca cells | essentially the same in each sex |

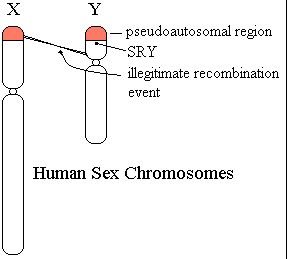
Although the human sex chromosomes are very different in size they are thought to be descended from an ancestral pair of chromosomes which were identical. One residue of this homology is the presence of regions of identity, near the two telomeres. The short arm region undergoes a genetic recombination event between X and Y once in every male meiosis, the long arm region also recombines but less frequently. These regions, because they are present in two recombining copies are known as pseudoautosomal regions. Very rarely, about once in 10,000 meioses, an illegitimate, unequal, crossover takes place with the exchange point on the Y chromosome located within the Y specific region as shown in the diagram. This leads to transfer of Y specific sequences to the paternal X chromosome. When this chromosome is inherited it causes the production of an XX male. i.e. a normal man, perhaps somewhat on the short size with height in the normal female range, with no abnormalities of sexual development. His only problem will be that he is infertile through lacking certain genes carried on the region of the Y chromosome which he does not possess.
| First, a word of warning. As you read the literature you will come across several candidates which were at one time promising but which proved to be false leads. Prominent among these are HY and a reptitive DNA sequence known as Bkm (Banded Krait Minor satellite). The experimental evidence on which both of these candidates (particularly HY) was based was extremely weak and was interpreted with a great deal of over optimism. |
Studying XX males and working out what was the smallest region of the Y chromosome required for male development (and similarly looking at the much rarer XY females with the reciprocal exchange product) led Page et al. to discover what they thought was the smallest region on The Y chromosome which contained the sex determining gene. Screening this region carefully they found a gene coding for a protein with 13 zinc fingers which they named (cautiously but wisely as it turned out) ZFY (Zinc Fingers, Y encoded).
After a year had gone by without proof that ZFY was indeed the testis determining factor TDF doubts began to be raised. Some of the factors which spoke against the role of ZFY were:
This last discovery led to the discovery of the gene SRY (Sex Region on the Y chromosome) This gene maps only 5kb to the male specific side of the PAB, it is intronless. It has three domains, the N terminal domain, the so called Sry box which resembles the DNA binding domain of the transcription factor HMG1, and a C terminal domain. Several lines of evidence proved that SRY was a sex determining gene.
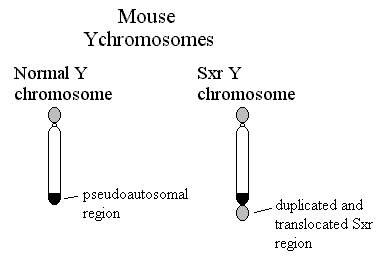
| Incidentally, Page et al were led astray by assuming that the deletion which had caused one of their XY females was a simple event. It was not, the patient was deleted for ZFY as they knew, but she had a second deletion which included SRY. |
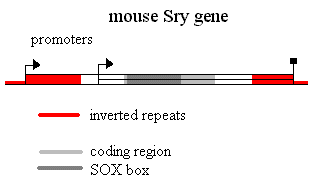
 The mouse
Sry gene is flanked by a long inverted repeat. It has alternative promoters, one
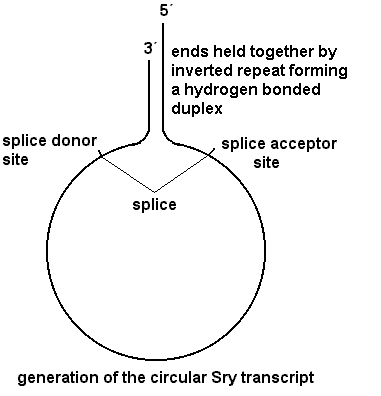
in the repeat and one closer to the gene. Splicing within the inverted repeat
(splice donor site in the 3'UTR, splice acceptor site in the 5'UTR) can give a
circular transcript which because it lacks a cap presumably cannot be
translated. The 3'UTR is full of sequences implicated in mRNA instability. The C
terminal domain is translated from a degenerate CAG repeat. It is thus very rich
in glutamines and histidines. The human SRY is completely different except in
the 79 amino acid SOX box which is 85% identical to the mouse. Sry genes are
found in all male mammals and there is no sequence conservation except in the
SOX box. It is one of the most rapidly evolving genes known. Almost all
mutations which cause XY females in humans are found in the SOX box. So most of
the function is in that box. The human gene does not work in mice so the
remaining structure may be of some importance. However, Mus musculus
domesticus has a truncated C terminal but still works on a Mus musculus
musculus background.
The mouse
Sry gene is flanked by a long inverted repeat. It has alternative promoters, one
in the repeat and one closer to the gene. Splicing within the inverted repeat
(splice donor site in the 3'UTR, splice acceptor site in the 5'UTR) can give a
circular transcript which because it lacks a cap presumably cannot be
translated. The 3'UTR is full of sequences implicated in mRNA instability. The C
terminal domain is translated from a degenerate CAG repeat. It is thus very rich
in glutamines and histidines. The human SRY is completely different except in
the 79 amino acid SOX box which is 85% identical to the mouse. Sry genes are
found in all male mammals and there is no sequence conservation except in the
SOX box. It is one of the most rapidly evolving genes known. Almost all
mutations which cause XY females in humans are found in the SOX box. So most of
the function is in that box. The human gene does not work in mice so the
remaining structure may be of some importance. However, Mus musculus
domesticus has a truncated C terminal but still works on a Mus musculus
musculus background.
The box binds in vitro to any bent (e.g. cruciform) DNA. But it binds with a higher affinity (to the minor groove of DNA) with some preferred sequences. The highest affinity is obtained with AACAAT but it recognises variants of this. When it binds it bends the DNA through almost 90º. Mutant SRYs which no longer confer maleness nevertheless still bind to cruciform DNA but no longer bind to AACAAT with the same high affinity.
SOX9 A disease which affects bone formation, campomelic dysplasia, is often accompanied by XY female sex reversal. It was originally thought to be an autosomal recessive. The gene was found when a patient with the disease and sex reversal was found to have a translocation of 17q and a gene related to SRY was found only 50kb away from the translocation breakpoint. Other patients were found in which there were mutations within this gene. Haploinsufficiency causes the bone malformations and also the sex reversal. Sox9 is expressed at 10.5 d.p.c. in the mouse in both sexes (shortly after the first appearance of Sry transcripts in males). It is then down regulated in females but its expression is increased in males. The gene is expressed in Sertoli cells. Interestingly, it is also expressed in the developing Sertoli cells of male chickens (which are chromosomally ZZ and which don't have a recognisable SRY). The Sox9 protein has been shown to regulate the expression of type II collagen directly (binding to a site in the first intron). The fact that it is rapidly down regulated in females suggests that there may be a repressor. Wunderle et al have generated transgenic mice containing SOX9-lacZ fusion genes with variable amounts of upstream sequences (from YACs). They have shown that regulatory elements are conserved between mouse and humans (these constructs of human DNA are properly regulated in the mouse). Also that rearrangements like those found in some campomelic dysplasic patients cause the gene not to function properly.
DAX1 Are there such things as ovary determining genes? One would predict that loss of function mutations in such genes would have no effect in males but that gain of function mutations would lead to XY sex reversal. These predictions would be the other way round in females. The syndrome known as Dosage Sensitive Sex reversal provides an example. Duplication of the chromosome band Xp21 gives rise to XY females. The minimum region of duplication (defined by examining a number of diferent overlapping duplications) is at most 160kb long. Within this region are several genes but one of them, DAX1, seems to be a good candidate. Deletion of this gene causes adrenal hypoplasia. The deletion does not cause any sex reversal in males. Dax1, the mouse gene, is expressed in developing gonads. It seems to be repressed by Sry and in its turn perhaps to repress Sox9. Over expression of Dax1 transgenes does not cause sex reversal in mouse except in the case of the 'weak' Y chromosome of Mus poschiavinus in a musculus background.
In conclusion, we do not know nearly as much about sex determination in mammals as we do about sex determination in Drosophila which has a fascinating cascade of genes which are differentially spliced in males and females. However, the first gene in the pathway, SRY, has been identified as has a good candidate for the next gene in the regulatory cascade, SOX9. DAX1 and other genes are clearly also involved in the process, though precisely where remains to be seen.